Approach
In flow battery technology, electrical energy is stored as chemical energy in electroactive species (e.g., vanadium species) that are dissolved in liquids (electrolytes). Energy conversion takes place in an electrochemical reactor having a positive and a negative electrode, while energy in the form of dissolved charged species is stored in two external reservoirs (for positive and negative species, respectively). In this way, power (size of the reactor) and energy (size of reservoirs) are independently scaled, which is one of the major advantages of this family of battery technologies.
However, having the energy-storing species dissolved in a liquid also constitutes an intrinsic drawback since maintaining dissolved species confined in the positive and negative compartments to avoid mixing becomes a tremendous challenge. Expensive ion-selective membranes (e.g. Nafion®) are commonly used to maintain the ionic connection of the positive and negative compartments while confining dissolved species on their corresponding side (Fig 1).
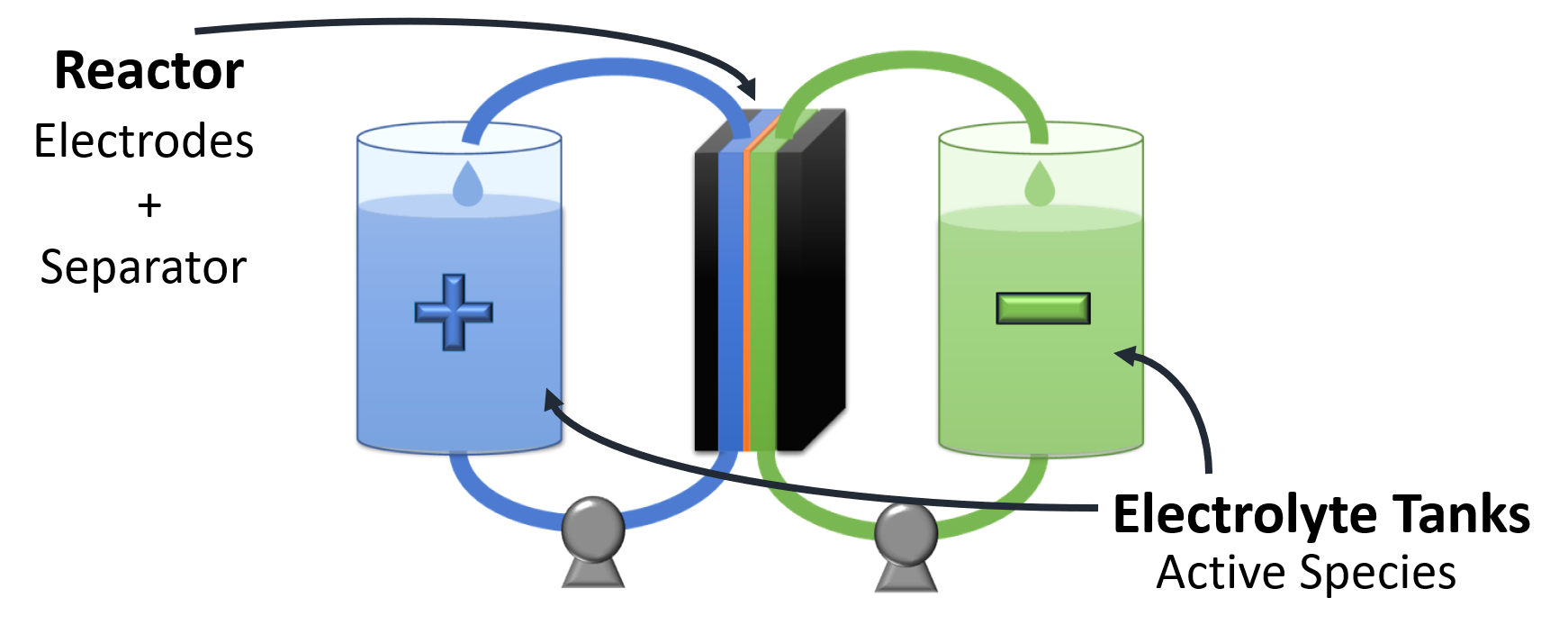
Figure 1. Scheme of a redox-flow battery.
However, the use of such expensive membranes is a not effective over long periods of time as it goes against thermodynamics: there is a chemical potential (concentration gradient) that drives species from one compartment to the other. In fact, since energy density (Wh L-1) is dependent on the concentration of species, highly concentrated solutions (> 1 M) are targeted, which generates a tremendous concentration gradient between the compartments. Over time, species end up crossing over as thermodynamics dictates, which leads to energy storage capacity fading.
Fortunately, all active species in the All-Vanadium Flow Battery Technology (AVFB) are vanadium-ions at different state of oxidation, which mitigates the issue of species cross-contamination over long periods as re-balancing of species is possible by simple periodic electrolyte remixing. However, crossover in emerging RFB technologies, e.g. organic flow battery (different species in each compartment), leads to irreversible losses, which illustrates the magnitude of the challenge of confining high concentrations of dissolved species “against thermodynamics”.
The science-towards-technology breakthrough of the proposed battery technology concept relies on making use of thermodynamically-driven concepts in a flowing configuration system. Mastering the thermodynamics and kinetics interfaces will enable us to envision a new flow battery technology with an excellent balance among the key performance indicators
